Management of Wagyu Recessive Genetic Conditions
The scientific terms for the two most common modes of genetic inheritance are: autosomal dominant and autosomal recessive. The term Autosomal means that the condition or trait has a genetic basis (controlled by genes). With cattle breeding a single allele (copy of a gene) comes from each parent for the seven billion genes in the cattle genome.
Autosomal dominant describes a trait or condition that is expressed when only a single allele (copy of a gene) is present. A practical example of this is the trait of black coat colour. Only one parent is required to input the dominant “black coat colour” allele to have a resultant black coloured calf.
Autosomal recessive describes a trait or condition that is expressed when two alleles are present (one from each parent). Two copies of the recessive allele are required as animals carrying only one copy of the recessive allele do not exhibit the trait (Carriers). A practical example of this is the recessive condition CL16. Animals with one copy of the recessive allele (Carriers) show no disease symptoms and appear normal. One copy of the recessive allele must be present from both parents to show symptoms of CL16 disease. Two copies of the recessive allele must be present before an animal shows symptoms of the recessive disease (Affected animal). Carriers appear phenotypically (observable characteristics) normal and are indistinguishable from non-carriers (Free animals) unless genetic testing is performed. Free animals inherit the normal gene from both parents.
Please Note that the importance of recessive testing is to identify carriers that may be used as breeding stock. If carrier sires are used as a terminal cross, the impact of harmful recessive conditions is diminished (assuming the breeding herd is a non-carrier or free (F) status) as it would be expected that all progeny is slaughtered.
Reported Wagyu Recessive Conditions
 |
Chediak Higashi Syndrome (CHS) status
CHS is a macrophage disorder (a white blood cell that has an important role in the immune response to disease). If cattle have a malfunctioning immune system, this makes them unable to resist bacterial challenge. Blood is slow to coagulate so often the first indicator is unusual umbilical cord haemorrhage at parturition (calving). Cattle with this syndrome often have an unusually pale coat colour. Photograph – Affected newborn calf with a pale coat |
 |
Spherocystosis (B3) status
This is a disorder of the surface membrane of the erythrocyte (red blood cells). The protein from the B3 gene makes up the basic structure of the erythrocyte. Cattle that are homozygous (have two copies of the recessive allele) have pernicious anaemia (bleeding caused by the abnormal red blood cells). Death normally occurs within the first 7 days after birth. Some cases live to adulthood but there is a severe retardation in growth. Photographs – Icterus in the eyes & anaemic calf @ 5m (90 kg) |
 |
Claudin 16 deficiency (CL16) status (Type 1)
Claudin 16 or RTD (Renal tubular dysplasia) is a gene disorder on chromosome 1 and causes kidney failure (chronic interstitial nephritis (CIN), often with zonal fibrosis or excess of fibrous connective tissue. There are 2 types of the deletion in this gene that can cause CIN. This disorder results in terminal kidney failure and the onset can occur any time from late adolescence. Cattle are unlikely to live more than 6 years. Photograph – Elongated Hooves on an Affected CL16 |
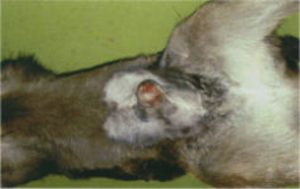 |
Factor 13 deficiency (F13) status
This gene contributes to fibrin stability, which is an integral part of the blood coagulation pathway (blood clotting ability). Disorders in this gene cause severe haemorrhage (bleeding). In calves, haemorrhage is particularly likely to occur in the hindquarters causing blood to pool and stagnate under the hide. In adult cattle any minor trauma (such as hitting the animal) can cause major haemorrhage at the trauma site. Note that no F13 Carriers have been identified in the tested US Wagyu population. Photograph – Navel of an Affected F13 newborn
|
|
|
Factor XI deficiency (F11) status (usually non-lethal)
Factor XI (F11) is a plasma protein that participates in the formation of blood clots. Factor XI deficiency is an autosomal disorder that is associated with mild bleeding in Wagyu. Affected animals show prolonged bleeding time and abnormal plasma coagulation after trauma or surgical procedures such as castration or dehorning. Photograph – Affected F11 Female exhibiting no harmful symptoms
|
*Photo source – Color Atlas of Bovine Congenital Defects (Ed. K.Hamana: 2006), GRS
Recessive Gene Status
DNA tests report the status of animal using three (3) categories:
- Free indicates that the sample submitted for this animal has been found to be clear of the causative mutation (abnormal gene) responsible for an identified autosomal condition. This animal is homozygous free, meaning that it has two copies of the normal allele.
- Carrier indicates that the animal has been found to have one copy of the causative mutation responsible for an identified autosomal condition. This animal is heterozygous for the mutation, meaning that it has one abnormal allele and one normal allele. This animal could pass the mutation (abnormal gene) to approximately half of its progeny.
- Affected calves are homozygous for the mutation responsible for the autosomal condition and have two copies of the abnormal variant of the gene.*Note that while unusual, some Affected (A) animals will reach breeding age and produce offspring
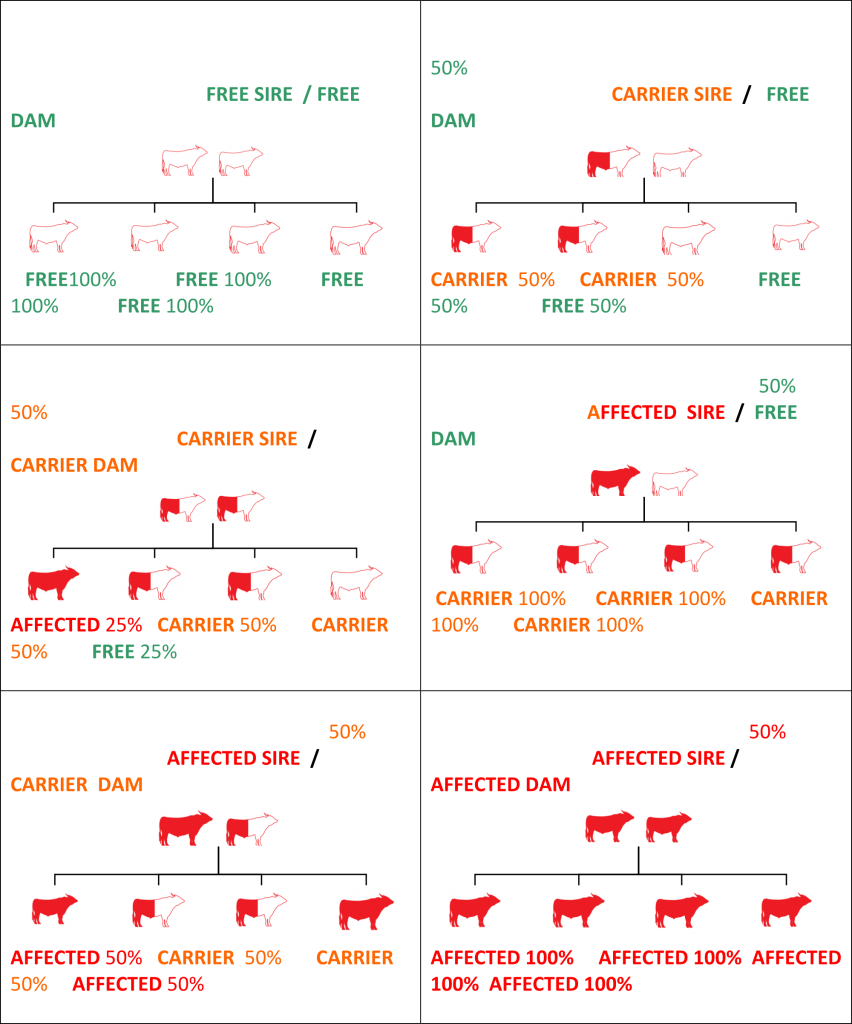
As one gene comes from each parent; expected progeny results of breeding different genetic condition status sires & dams are listed in table format belowWhat does this mean for management of the genetic conditions?
Figure 1
Dealing with recessive conditions
Unlike diseases that are the result of pathogenic agents such as bacteria or viruses that may result in heavy losses of animals and major de-contamination procedures, genetic conditions are not contagious and CANNOT be transmitted merely by animal-to-animal contact. Control of genetic conditions requires good management practices. These are developed based on the aims and objectives of each enterprise. Control of genetic conditions requires good management practices.
Management Options For Wagyu Recessive Conditions
Animals with a known carrier status can be used for breeding. However, follow-up testing of all progeny would be essential to develop future breeding strategies. When using Carrier animals for breeding, you can expect approximately 50% of the progeny to be Carriers for that particular condition. Carrier animals can be culled, separated or used with care. The remaining progeny will be Free animals and can be used to perpetuate the herd. Continued breeding by joining two Carrier animals with the same condition is not recommended due to the risk of producing Affected animals.
The important thing to remember with autosomal recessive conditions is that they are a challenge to be managed and not feared.
Many management options exist for the control of genetic conditions and these are formulated based on the individual needs and requirements of each enterprise. Some of these include:
- Test all animals and remove Carriers.
- Test all animals and use Carriers ONLY in terminal breeding programs.
- Test sires and only use Free bulls for breeding.
- Use Carriers for breeding ONLY on Free animals. Test all progeny.
- Use Carriers for breeding on animals that are Free of the same condition. (Example – Use F11 Carriers on F11 Free animals).
What about cross-breeds?
Recessive conditions can be perpetuated in cross-breeds. First crosses are unlikely to produce any Affected progeny (unless both breeds have the same condition). Subsequent inter-breeding of cross-bred animals may produce additional Carriers and Affected animals.
Can you retain superior production genetics from carrier animals?
Yes! Carrier sires and dams can be used for breeding. However, follow-up testing of all progeny is essential for future breeding strategies with that progeny. Expect 50% of their progeny to be Carriers. These Carriers can be identified by testing. Breeding by joining two Carriers (with the same condition) is not recommended due to the risk of producing Affected animals.
Source:
1. Marsh, I and Galea, F – Industry and Investment, NSW Elizabeth Macarthur Agricultural Institute (EMAI) Australia
2. Hirayama, H., S. Kageyama, S. Moriyasu, T. Hirano, Y. Sugimoto, N. Kobayashi, M. Inaba, K. Sawai, S. Onoe, and A. Minamihashi. 2004. Genetic diagnosis of claudin-16 defciency and sex determination in bovine preimplantation embryos. J Reprod Dev 50:613-8.2. Kageyama, S., H. Hirayama, S. Moriyasu, M. Inaba, D. Ito, H. Ohta, K. Sawai, A. Minamihashi, and S. Onoe. 2006. Genetic diagnosis of band 3 deficiency and sexing in bovine preimplantation embryos. J Vet Med Sci 68:319-23.3. Kunieda, M., T. Tsuji, A. R. Abbasi, M. Khalaj, M. Ikeda, K. Miyadera, H. Ogawa, and T. Kunieda. 2005. An insertion mutation of the bovine Fii gene is responsible for factor XI defciency in Japanese black cattle. Mamm Genome 16:383-9.4. Kunieda, T., M. Nakagiri, M. Takami, H. Ide, and H. Ogawa. 1999. Cloning of bovine LYST gene and identifcation of a missense mutation associated with Chediak-Higashi syndrome of cattle. Mamm Genome 10:1146-9.

